Titanium dioxide
| Titanium dioxide | |
|---|---|
_oxide.jpg) |
|
 |
|
|
Titanium dioxide
Titanium(IV) oxide |
|
|
Other names
Titania
Rutile Anatase Brookite |
|
| Identifiers | |
| CAS number | 13463-67-7 |
| PubChem | 26042 |
| RTECS number | XR2775000 |
| Properties | |
| Molecular formula | TiO2 |
| Molar mass | 79.866 g/mol |
| Appearance | White solid |
| Density | 4.23 g/cm3 |
| Melting point |
1843 °C |
| Boiling point |
2972 °C |
| Refractive index (nD) | 2.488 (anatase) 2.583 (brookite) 2.609 (rutile) |
| Hazards | |
| MSDS | ICSC 0338 |
| EU classification | Not listed |
| NFPA 704 |
 0
1
0
|
| Flash point | Non-flammable |
| Related compounds | |
| Other cations | Zirconium dioxide Hafnium dioxide |
| Related titanium oxides | Titanium(II) oxide Titanium(III) oxide Titanium(III,IV) oxide |
| Related compounds | Titanic acid |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Titanium dioxide, also known as titanium(IV) oxide or titania, is the naturally occurring oxide of titanium, chemical formula TiO2. When used as a pigment, it is called titanium white, Pigment White 6, or CI 77891. It is noteworthy for its wide range of applications, from paint to sunscreen to food colouring, for which it was given E number E171.
Contents |
Occurrence
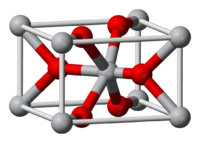
Titanium dioxide occurs in nature as well-known minerals rutile, anatase and brookite, and additionally as two high pressure forms, a monoclinic baddeleyite-like form and an orthorhombic α-PbO2-like form, both found recently at the Ries crater in Bavaria.[1][2] The most common form is rutile,[3] which is also the most stable form. Anatase and brookite both convert to rutile upon heating.[3] Rutile, anatase and brookite all contain six coordinated titanium.
Titanium dioxide has eight modifications - in addition to rutile, anatase and brookite there are three metastable forms produced synthetically (monoclinic, tetragonal and orthorombic), and five high pressure forms (α-PbO2-like, baddeleyite-like and cotunnite-like):
| Form | Crystal system | Synthesis |
|---|---|---|
| rutile | tetragonal | |
| anatase | tetragonal | |
| brookite | orthorhombic | |
| TiO2(B)[4] | monoclinic | Hydrolysis of K2Ti4O9 followed by heating |
| TiO2(H), hollandite-like form[5] | tetragonal | Oxidation of the related potassium titanate bronze, K0.25TiO2 |
| TiO2(R), ramsdellite-like form[6] | orthorhombic | Oxidation of the related lithium titanate bronze Li0.5TiO2 |
| TiO2(II)-(α-PbO2-like form)[7] | orthorhombic | |
| baddeleyite-like form, (7 coordinated Ti)[8] | monoclinic | |
| TiO2 -OI[9] | orthorhombic | |
| cubic form[10] | cubic | |
| TiO2 -OII, cotunnite(PbCl2)-like[11] | orthorhombic |
The naturally occurring oxides can be mined and serve as a source for commercial titanium. The metal can also be mined from other minerals such as ilmenite or leucoxene ores, or one of the purest forms, rutile beach sand. Star sapphires and rubies get their asterism from rutile impurities present in them.[12]
Titanium dioxide (B) is found as a mineral in weathering rims on tektites and perovskite and as lamellae in anatase from hydrothermal veins and has a relatively low density.[13]
Spectral lines from titanium oxide are prominent in class M stars, which are cool enough to allow molecules of this chemical to form.
Production
Crude titanium dioxide is purified via converting to titanium tetrachloride in the chloride process. In this process, the crude ore (containing at least 70% TiO2) is reduced with carbon, oxidized with chlorine to give titanium tetrachloride; i.e., carbothermal chlorination. This titanium tetrachloride is distilled, and re-oxidized in a pure oxygen flame or plasma at 1500–2000 K to give pure titanium dioxide while also regenerating chlorine.[14] Aluminium chloride is often added to the process as a rutile promotor; the product is mostly anatase in its absence.
Another widely used process utilizes ilmenite as the titanium dioxide source, which is digested in sulfuric acid. The by-product iron(II) sulfate is crystallized and filtered-off to yield only the titanium salt in the digestion solution, which is processed further to give pure titanium dioxide. Another method for upgrading ilmenite is called the Becher Process. One method for the production of titanium dioxide with relevance to nanotechnology is solvothermal Synthesis of titanium dioxide.
In the laboratory, anatase can be converted in a hydrothermal synthesis to delaminated anatase inorganic nanotubes [15] and titanate nanoribbons which are of potential interest as catalytic supports and photocatalysts. For this to happen, anatase is mixed with 10M sodium hydroxide and heated at 130 °C for 72 hours. The reaction product is washed with dilute hydrochloric acid and heated at 400 °C for another 15 hours. the yield of nanotubes is quantitative and the tubes have an outer diameter of 10 to 20 nanometers and an inner diameter of 5 to 8 nanometers and have a length of 1 micron. A higher reaction temperature (170 °C) and less reaction volume gives the corresponding nanowires.[16]
Applications
Pigment
Titanium dioxide is the most widely used white pigment because of its brightness and very high refractive index (n = 2.7), in which it is surpassed only by a few other materials. Approximately 4 million tons of pigmentary TiO2 are consumed annually worldwide. When deposited as a thin film, its refractive index and colour make it an excellent reflective optical coating for dielectric mirrors and some gemstones like "mystic fire topaz". TiO2 is also an effective opacifier in powder form, where it is employed as a pigment to provide whiteness and opacity to products such as paints, coatings, plastics, papers, inks, foods, medicines (i.e. pills and tablets) as well as most toothpastes. Opacity is improved by optimal sizing of the titanium dioxide particles.
Used as a white food colouring, it has E number E171. Titanium dioxide is often used to whiten skimmed milk; this has been shown statistically to increase skimmed milk's palatability.[17]
In cosmetic and skin care products, titanium dioxide is used as a pigment, sunscreen and a thickener. It is also used as a tattoo pigment and in styptic pencils. Titanium dioxide is produced in varying particle sizes, oil and water dispersable, and with varying coatings for the cosmetic industry. This pigment is used extensively in plastics and other applications for its UV resistant properties where it acts as a UV absorber, efficiently transforming destructive UV light energy into heat.
In ceramic glazes titanium dioxide acts as an opacifier and seeds crystal formation.
Titanium dioxide is found in almost every sunscreen with a physical blocker because of its high refractive index, its strong UV light absorbing capabilities and its resistance to discolouration under ultraviolet light. This advantage enhances its stability and ability to protect the skin from ultraviolet light. Sunscreens designed for infants or people with sensitive skin are often based on titanium dioxide and/or zinc oxide, as these mineral UV blockers are believed to cause less skin irritation than chemical UV absorber ingredients. The titanium dioxide particles used in sunscreens have to be coated with silica or alumina, because titanium dioxide creates radicals in the photocatalytic reaction. These radicals are carcinogenic, and could damage the skin.
Titanium dioxide is used to mark the white lines on the tennis courts of the All England Lawn Tennis and Croquet Club, best known as the venue for the annual grand slam tennis tournament The Championships, Wimbledon.[18]
The exterior of the Saturn V rocket was painted with titanium dioxide; this later allowed astronomers to determine that J002E3 was the S-IVB stage from Apollo 12 and not an asteroid.
As a photocatalyst

Titanium dioxide, particularly in the anatase form, is a photocatalyst under ultraviolet (UV) light. Recently it has been found that titanium dioxide, when spiked with nitrogen ions or doped with metal oxide like tungsten trioxide, is also a photocatalyst under either visible or UV light. The strong oxidative potential of the positive holes oxidizes water to create hydroxyl radicals. It can also oxidize oxygen or organic materials directly. Titanium dioxide is thus added to paints, cements, windows, tiles, or other products for its sterilizing, deodorizing and anti-fouling properties and is used as a hydrolysis catalyst. It is also used in the Graetzel cell, a type of chemical solar cell.
The photocatalytic properties of titanium dioxide were discovered by Akira Fujishima in 1967[19] and published in 1972.[20] The process on the surface of the titanium dioxide was called the Honda-Fujishima effect.[19] Titanium dioxide has potential for use in energy production: as a photocatalyst, it can
- carry out hydrolysis; i.e., break water into hydrogen and oxygen. Were the hydrogen collected, it could be used as a fuel. The efficiency of this process can be greatly improved by doping the oxide with carbon.[21]
- Titanium dioxide can also produce electricity when in nanoparticle form. Research suggests that by using these nanoparticles to form the pixels of a screen, they generate electricity when transparent and under the influence of light. If subjected to electricity on the other hand, the nanoparticles blacken, forming the basic characteristics of a LCD screen. According to creator Zoran Radivojevic, Nokia has already built a functional 200-by-200-pixel monochromatic screen which is energetically self-sufficient.
In 1995 Fujishima and his group discovered the superhydrophilicity phenomenon for titanium dioxide coated glass exposed to sun light.[19] This resulted in the development of self-cleaning glass and anti-fogging coatings.
TiO2 incorporated into outdoor building materials, such as paving stones in noxer blocks or paints, can substantially reduce concentrations of airborne pollutants such as volatile organic compounds and nitrogen oxides.[22]
A photocatalytic cement that uses titanium dioxide as a primary component, produced by Italcementi Group, was included in Time's Top 50 Inventions of 2008.[23]
TiO2 offers great potential as an industrial technology for detoxification or remediation of wastewater due to several factors.
- The process occurs under ambient conditions very slowly; direct UV light exposure increases the rate of reaction.
- The formation of photocyclized intermediate products, unlike direct photolysis techniques, is avoided.
- Oxidation of the substrates to CO2 is complete.
- The photocatalyst is inexpensive and has a high turnover.
- TiO2 can be supported on suitable reactor substrates.
As a storage medium
Researchers at the University of Tokyo, Japan have created a 25 terabyte titanium oxide-based disc.[24]
Other applications
It is also used in resistance-type lambda probes (a type of oxygen sensor).
Titanium dioxide is what allows osseointegration between an artificial medical implant and bone.
Titanium dioxide in solution or suspension can be used to cleave protein that contains the amino acid proline at the site where proline is present. This breakthrough in cost-effective protein splitting took place at Arizona State University in 2006.[25]
Titanium dioxide on silica is being developed as a form of odor control in cat litter. The photocatalyst is vastly cheaper than silica beads per usage and effectively eliminates odor for longer.
Titanium dioxide is also used as a material in the memristor, a new electronic circuit element. It can be employed for solar energy conversion based on dye, polymer, or quantum dot sensitized nanocrystalline TiO2 solar cells using conjugated polymers as solid electrolytes.[26]
It has also been recently incorporated as a photocatalyst into dental bleaching products. It allows the use of decreased concentrations of hydrogen peroxide in the bleaching agent, thus claimed to achieve similar bleaching effects with fewer side effects (e.g. transient sensitivity, change in tooth surface topography, etc.)
It is also used by film and television companies as a substitute for snow when filming scenes which require a winter setting.
Synthetic single crystals and films of TiO2 are used as a semiconductor,[27] and also in Bragg-stack style dielectric mirrors due to the high refractive index of TiO2 (2.5 – 2.9).[28][29]
Health and safety
Titanium dioxide is incompatible with strong oxidizers and strong acids.[30] Violent or incandescent reactions may occur with metals (e.g. aluminium, calcium, magnesium, potassium, sodium, zinc and lithium).[31]
Titanium dioxide dust, when inhaled, has recently been classified by the International Agency for Research on Cancer (IARC) as an IARC Group 2B carcinogen possibly carcinogenic to humans.[32] Titanium dioxide accounts for 70% of the total production volume of pigments worldwide. It is widely used to provide whiteness and opacity to products such as paints, plastics, papers, inks, foods, and toothpastes. It is also used in cosmetic and skin care products, and it is present in almost every sunblock, where it helps protect the skin from ultraviolet light.
Many sunscreens use nano particle titanium dioxide (along with nano particle zinc oxide) which does get absorbed into the skin.[33][34] This could cause as of yet unknown health problems.[35]
The findings of the IARC are based on the discovery that high concentrations of pigment-grade (powdered) and ultrafine titanium dioxide dust caused respiratory tract cancer in rats exposed by inhalation and intratracheal instillation.[36] The series of biological events or steps that produce the rat lung cancers (e.g. particle deposition, impaired lung clearance, cell injury, fibrosis, mutations and ultimately cancer) have also been seen in people working in dusty environments. Therefore, the observations of cancer in animals were considered, by IARC, as relevant to people doing jobs with exposures to titanium dioxide dust. For example, titanium dioxide production workers may be exposed to high dust concentrations during packing, milling, site cleaning and maintenance, if there are insufficient dust control measures in place. However, it should be noted that the human studies conducted so far do not suggest an association between occupational exposure to titanium dioxide and an increased risk for cancer. The safety of the use of nano-particle sized Titanium Dioxide, which can penetrate the body and reach internal organs, has been criticized.[37] Studies have also found that titanium dioxide nanoparticles cause genetic damage in mice, suggesting that humans may be at risk of cancer or genetic disorders resulting from exposure.[38]
See also
- Dye-sensitized solar cell
- Noxer, a building material incorporating TiO2.
- Timeline of hydrogen technologies
References
- ↑ El, Goresy; Chen, M; Dubrovinsky, L; Gillet, P; Graup, G (2001). "An ultradense polymorph of rutile with seven-coordinated titanium from the Ries crater.". Science 293 (5534): 1467–70. doi:10.1126/science.1062342. PMID 11520981.
- ↑ El Goresy, Ahmed (2001). "A natural shock-induced dense polymorph of rutile with α-PbO2 structure in the suevite from the Ries crater in Germany". Earth and Planetary Science Letters 192: 485. doi:10.1016/S0012-821X(01)00480-0.
- ↑ 3.0 3.1 Greenwood, Norman N.; Earnshaw, A. (1984), Chemistry of the Elements, Oxford: Pergamon, pp. 1117–19, ISBN 0-08-022057-6
- ↑ Marchand R., Brohan L., Tournoux M. (1980). "A new form of titanium dioxide and the potassium octatitanate K2Ti8O17". Materials Research Bulletin 15 (8): 1129–1133. doi:10.1016/0025-5408(80)90076-8.
- ↑ Latroche, M; Brohan, L; Marchand, R; Tournoux, (1989). "New hollandite oxides: TiO2(H) and K0.06TiO2". Journal of Solid State Chemistry 81 (1): 78–82. doi:10.1016/0022-4596(89)90204-1.
- ↑ J. Akimoto, Y. Gotoh, Y. Oosawa, N. Nonose, T. Kumagai, K. Aoki, H. Takei (1994). "Topotactic Oxidation of Ramsdellite-Type Li0.5TiO2, a New Polymorph of Titanium Dioxide: TiO2(R)". Journal of Solid State Chemistry 113 (1): 27–36. doi:10.1006/jssc.1994.1337.
- ↑ P. Y. Simons, F. Dachille (1967). "The structure of TiO2II, a high-pressure phase of TiO2". Acta Crystallographica 23 (2): 334–336. doi:10.1107/S0365110X67002713.
- ↑ Sato H. , Endo S, Sugiyama M, Kikegawa T, Shimomura O, Kusaba K (1991). "Baddeleyite-Type High-Pressure Phase of TiO2". Science 251 (4995): 786 – 788. doi:10.1126/science.251.4995.786. PMID 17775458.
- ↑ Dubrovinskaia N A, Dubrovinsky L S., Ahuja R, Prokopenko V B., Dmitriev V., Weber H.-P., Osorio-Guillen J. M., Johansson B (2001). "Experimental and Theoretical Identification of a New High-Pressure TiO2 Polymorph.". Phys. Rev. Lett. 87: 275501. doi:10.1103/PhysRevLett.87.275501.
- ↑ Mattesini M, de Almeida J. S., Dubrovinsky L., Dubrovinskaia L, Johansson B., Ahuja R. (2004). "High-pressure and high-temperature synthesis of the cubic TiO2 polymorph". Phys. Rev. B 70: 212101. doi:10.1103/PhysRevB.70.212101.
- ↑ Dubrovinsky, LS; Dubrovinskaia, NA; Swamy, V; Muscat, J; Harrison, NM; Ahuja, R; Holm, B; Johansson, B (2001). "Materials science: The hardest known oxide". Nature 410 (6829): 653–654. doi:10.1038/35070650. PMID 11287944.
- ↑ Emsley, John (2001). Nature's Building Blocks: An A–Z Guide to the Elements. Oxford: Oxford University Press. pp. 451–53. ISBN 0-19-850341-5.
- ↑ Banfield, J. F., Veblen, D. R., and Smith, D. J. (1991). "The identification of naturally occurring TiO2 (B) by structure determination using high-resolution electron microscopy, image simulation, and distance–least–squares refinement". American Mineralogist 76: 343.
- ↑ "Titanium Dioxide Manufacturing Processes". Millennium Inorganic Chemicals. http://www.millenniumchem.com/Products+and+Services/Products+by+Type/Titanium+Dioxide+-+Paint+and+Coatings/r_TiO2+Fundamentals/Titanium+Dioxide+Manufacturing+Processes_EN.htm. Retrieved 2007-09-05.
- ↑ Gregory Mogilevsky, Qiang Chen, Alfred Kleinhammes, Yue Wu, The structure of multilayered titania nanotubes based on delaminated anatase, Chemical Physics Letters, Volume 460, Issues 4-6, 30 July 2008, Pages 517-520, ISSN 0009-2614, DOI: 10.1016/j.cplett.2008.06.063. (http://www.sciencedirect.com/science/article/B6TFN-4SVKST5-4/2/d58f9d2dcecde18150898ff641610a1d)
- ↑ Graham Armstrong, A. Robert Armstrong, Jesús Canales and Peter G. Bruce (2005). "Nanotubes with the TiO2-B structure". Chemical Communications: 2454. http://www.rsc.org/publishing/journals/CC/article.asp?doi=b501883h.
- ↑ Lance G. Phillips and David M. Barbano. "The Influence of Fat Substitutes Based on Protein and Titanium Dioxide on the Sensory Properties of Lowfat Milk". Journal of Dairy Science 80 (11): 2726. http://jds.fass.org/cgi/content/abstract/80/11/2726.
- ↑ "Light spells doom for bacteria". http://www.photonics.com/Content/ReadArticle.aspx?ArticleID=35722.
- ↑ 19.0 19.1 19.2 "Japan Nanonet Bulletin - 44th Issue - May 12, 2005: Discovery and applications of photocatalysis —Creating a comfortable future by making use of light energy"
- ↑ Fujishima, AKIRA (1972). "Electrochemical Photolysis of Water at a Semiconductor Electrode". Nature 238: 37. doi:10.1038/238037a0.
- ↑ Carbon-doped titanium dioxide is an effective photocatalyst
- ↑ "Smog-busting paint soaks up noxious gases", Jenny Hogan, 'newscientist.com, February 4, 2004
- ↑ TIME's Best Inventions of 2008, October 31, 2008
- ↑ "Titanium Oxide for High-Density Data Storage". Research. http://www.redorbit.com/news/technology/1869698/new_disc_could_hold_a_thousand_times_more_data/. Retrieved May 24, 2010.
- ↑ Jones, BJ; Vergne, MJ; Bunk, DM; Locascio, LE; Hayes, MA (2007). "Cleavage of Peptides and Proteins Using Light-Generated Radicals from Titanium Dioxide". Anal. Chem. 79 (4): 1327–1332. doi:10.1021/ac0613737. PMID 17297930.
- ↑ Lewis, Nathan. "Nanocrystalline TiO2". Research. California Institute of Technology. http://nsl.caltech.edu/research.nt.html. Retrieved October 9, 2009.
- ↑ M. D. Earle (1942). "The Electrical Conductivity of Titanium Dioxide". Physical Review 61 (1-2): 56. doi:10.1103/PhysRev.61.56.
- ↑ Paschotta, Rüdiger. "Bragg Mirrors". Encyclopedia of Laser Physics and Technology. RP Photonics. http://www.rp-photonics.com/bragg_mirrors.html. Retrieved May 1, 2009.
- ↑ Handbook of Chemistry and Physics (89 ed.). 2008–2009.
- ↑ Occupational Health Services, Inc. (31 May 1988). "Hazardline" (Electronic Bulletin). New York: Occupational Health Services, Inc..
- ↑ Sax, N.I.; Richard J. Lewis, Sr. (2000). Dangerous Properties of Industrial Materials. III (10th ed.). New York: Van Nostrand Reinhold. p. 3279. ISBN 978-0471354079.
- ↑ "Titanium dioxide". International Agency for Research on Cancer. 2006. http://monographs.iarc.fr/ENG/Meetings/93-titaniumdioxide.pdf.
- ↑ [http://www.foe.org/sites/default/files/SunscreensReport.pdf "Manufactured Nanomaterials and Sunscreens: Top Reasons for Precaution"]. August 19, 2009. http://www.foe.org/sites/default/files/SunscreensReport.pdf. Retrieved April 12, 2010.
- ↑ "Nano-tech sunscreen presents potential health risk". ABC News. December 18, 2008. http://www.abc.net.au/news/stories/2008/12/18/2450030.htm. Retrieved April 12, 2010.
- ↑ "Nano World: Nanoparticle toxicity tests". Physorg.com. April 5, 2006. http://www.physorg.com/news63466994.html. Retrieved April 12, 2010.
- ↑ Kutal, C., Serpone, N. (1993). Photosensitive Metal Organic Systems: Mechanistic Principles and Applications. American Chemical Society, Washington D.C.
- ↑ "Suncream may be linked to Alzheimer's disease, say experts". 24th August 2009. http://www.dailymail.co.uk/health/article-1208720/Suncream-linked-Alzheimers-disease-say-experts.html. Retrieved 2009-08-25.
- ↑ "Nanoparticles Used in Common Household Items Cause Genetic Damage in Mice". 17th November 2009. http://www.sciencedaily.com/releases/2009/11/091116165739.htm. Retrieved 2009-11-17.
External links
- International Chemical Safety Card 0338
- "Nano-Oxides, Inc. - Nano Powders, LEGIT information on Titanium Dioxide TiO2". www.nano-oxides.com. http://www.nano-oxides.com/pdf/TiO2_Brochure.pdf. Retrieved November2008.
- NIOSH Pocket Guide to Chemical Hazards
- "Fresh doubt over America map", bbc.co.uk, 30 July 2002
- Titanium Dioxide Classified as Possibly Carcinogenic to Humans, 2007 (if inhaled as a powder)
- A description of TiO2 photocatalysis
- Crystal structures of the three forms of TiO2
- "Architecture in Italy goes green", Elisabetta Povoledo, International Herald Tribune, November 22, 2006
- "A Concrete Step Toward Cleaner Air", Bruno Giussani, BusinessWeek.com, November 8, 2006
- "Titanium Dioxide Classified as Possibly Carcinogenic to Humans", Canadian Centre for Occupational Health and Safety, August, 2006
|
|||||
|
|||||||||||||||||